- Startseite
- Forschung
- Arbeitsgruppen & Labore
- AG Lokale Immunmodulation
AG Lokale Immunmodulation
Das Forschungsgebiet von Prof. Dr. rer. nat. Felix Bock ist die immunologische Mikro-Umgebung im Bereich des Hornhauttransplantats sowie die Wirkung immun-modulatorischer Moleküle auf das Überleben von Hornhauttransplantaten.
Immunvermittelte Transplantatabstoßungen sind nach wie vor die häufigste Ursache für Transplantatversagen nach Organ- und Gewebetransplantationen. Es besteht ein großer medizinischer Bedarf an pharmakologischen Strategien, die das Überleben des Transplantats fördern, ohne die Gesundheit des Empfängers übermäßig zu beeinträchtigen.
Der Goldstandard zur Hemmung der Immunreaktion, die zum Versagen des Hornhauttransplantats führt, ist die Verwendung von Kortikosteroiden. Dies funktioniert jedoch häufig nicht, und die topische oder systemische Anwendung dieser Immunsuppressiva führt zu schweren Nebenwirkungen wie Katarakt, Glaukom, Bindehautnekrose oder Wundheilungsstörungen. Andere, wie Cyclosporin A, haben keine signifikante Verbesserung des Transplantatüberlebens und keine gute Hornhautpenetration gezeigt. Daher besteht ein großer, bisher nicht ausreichend gedeckter Bedarf an der Entwicklung präziserer Therapien zur Förderung des Überlebens von Hornhauttransplantaten unter Vermeidung von Nebenwirkungen und zur Steigerung der therapeutischen Wirksamkeit. Unsere Forschung zum Verständnis der zugrundeliegenden immunologischen Prozesse in der Mikroumgebung von Transplantat und Empfänger wird zur Entwicklung solcher neuen Strategien beitragen.
On-site Mode of Action of locally applied immune modulatory factors
The pathological ingrowth of blood and lymphatic vessels (hem- and lymphangiogenesis) interferes with corneal transparency. The blood vessels themselves as well as collateral tissue alterations reduce the visual function up to blindness. Corneal (lymph)angiogenesis is associated with both the most frequent cause for corneal blindness worldwide (trachoma) as well as with the most frequent reason for the reduction of corneal visual acuity in developed nations (herpetic keratitis).
Pathological corneal hemangiogenesis not only reduces vision but also leads to a significant increase of the risk of an immune reaction after subsequent corneal transplantation. Grafts transplanted into an avascular corneal recipient bed (so called normal risk keratoplasty) show a 5-year survival rate of over 90%. In contrast, grafts implanted into prevascularized corneal recipient beds (high risk keratoplasty) are rejected in more than 50% despite of immunosuppressive therapy.
To support corneal graft survival upon transplantation, many therapeutics target VEGF-signaling which is the main driver for vascularization-dependent graft failure. VEGF-A not only regulates angiogenesis, but is known as a modulator of the immune microenvironment and plays an essential role in several immunological processes. In cancer research, VEGF is long known as local modulator of antigen-presenting cells, i.e. dendritic cells. Furthermore, it has been reported that overexpression of VEGF leads to a reduction in cytokine expression, while depletion of free VEGF can increase cytokine concentrations.
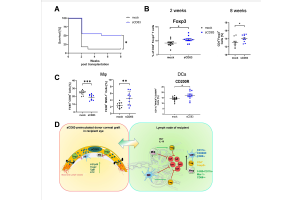
Induction of graft derived transplant tolerance
Derived from the well known term of “direct allo sensitivity” this project focuses on the induction of a so called “direct allo tolerance”.
In cooperation with Prof. Steinkasserer from Erlangen, we analyse in this project, if corneal graft tolerance can be mediated directly by immune cells of the donor tissue. Using the cornea has the benefit that not only the graft but also the recipient bed can be treated locally with immune modulators like sCD83. Thereby the effect of sCD83 on the immune cells of the graft as well as on the immunological micro-environment of the recipient bed can be analysed. Recently, we could show that preincubation of the donor cornea with sCD83 can improve graft survival via the induction of regulatory APCs and T cells. (Peckert-Maier K, Schönberg A, Wild AB, Royzman D, Braun G, Stich L, Hadrian K, Tripal P, Cursiefen C, Steinkasserer A, Zinser E, Bock F., Pre-incubation of corneal donor tissue with sCD83 improves graft survival via the induction of alternatively activated macrophages and tolerogenic dendritic cells. Am J Transplant, 2021).
In a similar project we could show that the already clinically used Aflibercept is also suitable for preincubation of the donor tissue and can improve graft survival. This approach is currently translated into the clinic. (Zhang W, Schönberg A, Hamdorf M, Georgiev T, Cursiefen C, Bock F. Preincubation of donor tissue with a VEGF cytokine trap promotes subsequent high-risk corneal transplant survival. Br J Ophthalmol. 2022)
This new therapeutic concept of transplant-mediated graft-tolerance induction could be the seeding point for new therapeutic approaches for other tissue- and solid organ transplantations like skin- or kidney transplantations in which a pre-treatment of the graft by immune modulators like sCD83 could also lead to tolerance induction against the donor tissue and avoid live long immunosuppressive treatment of the patient.
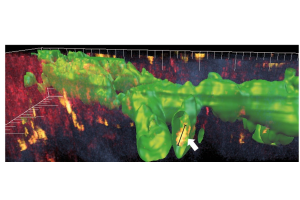
Immune cells (arrow, yellow) in Alexa488-labeled lymphatic vessels (green) could be observed by 3D reconstruction of a 2-photon image stack through the vascularized cornea (Steven, P.*, F. Bock*, G. Huttmann, and C. Cursiefen, Intravital two-photon microscopy of immune cell dynamics in corneal lymphatic vessels. PLoS One, 2011,10.1371/journal.pone.0026253.)
Spatial analysis of corneal immune cells in steady state or during inflammation by Histo-Cytometry
Nowadays it is widely accepted that the cornea contains antigen-presenting cells like macrophages, dendritic cells and Langerhans cells. We were able to show that the presence of myeloid cells, especially macrophages and activated DCs in the cornea significantly increase the risk of corneal transplant rejection. Characterization of these cells therefore provides important information about the immunological status of the cornea which helps to assess therapeutic strategies to improve disease outcomes.
Flow cytometry is a well-established and powerful tool for quantitative analysis of complex cell populations.It enables identification of distinct subpopulations and specific cell types by up to 17 parameters at once. But regarding the cornea it requires not only the tissue’s digestion but also the pooling of several corneas. Thus, there is an urgent need for reduction. Compared to flow cytometry, conventional microscopy is limited in quantification of specific cell subsets with defined marker combinations but provides information about the population’s spatial characteristics. Since during histological preparation of the specimens no loss of material/cells occurs, much less animals are needed for quantitative analysis.
The objective of the project is to characterize specific subsets of antigen presenting cells in the cornea in steady state or during inflammation directly in their microenvironment including spatial information. The Histo-Cytometry technique not only reduce significantly the amount of mice needed but also opens new possibilities for characterization of corneal immune cells in steady state or during inflammation in terms of their localization within the tissue.
Funding
DFG Research Unit 2240 (www.for2240.de): BO4489/1-2, HO 6270/1-1; BO 4489/3-1; EU Restore Vision (www.restorevision-project.eu; CC, FB); RETINOVIT Foundation Cologne; Köln Fortune Projekt Nr. 91/2023.