- Startseite
- Forschung
- Cornea Lab
Cornea Lab
Die Arbeitsgruppe um Prof. Dr. Claus Cursiefen und Prof. Dr. Felix Bock beschäftigt sich mit den Mechanismen der Transplantatabstoßung nach Hornhaut-Transplantation. Hierbei wird der Focus vor allem auf die Rolle der Lymphgefäße gelegt. Die Hornhaut besitzt ein sogenanntes „angiogenes Privileg“, wodurch die gesunde Hornhaut von Blut- und Lymphgefäßen freigehalten wird. Dieses System ist durch viele molekulare Mechanismen gestützt, die selbst noch greifen, wenn invasive Therapien wie Refraktive Chirurgie an der Hornhaut angewandt werden.
Dennoch kann es durch Infektionen, Verbrennungen oder auch durch vorangegangene Transplantationen zum Einwachsen von klinisch sichtbaren Blutgefäßen und klinisch unsichtbaren Lymphgefäßen kommen. Unsere Arbeitsgruppe untersucht mit verschiedenen Techniken sowohl die Entstehung und Interaktion der Lymphgefäße mit anderen Zellen des Immunsystems als auch Möglichkeiten, das Wachstum und die Interaktionen zu beeinflussen oder zu verhindern.
Angewandte Methoden sind die Immunhistochemie, Molekularbiologische Methoden wie qPCR und Westen Blot, FACS Analyse, Zellkultur und intravitale Multiphotonenmikroskopie in Zusammenarbeit mit der AG Augenoberfläche.
Die Arbeitsgruppe steht in engem Kontakt zur Klinik und ist daher stark in den Prozess "From Bench to Bedside and back" eingebunden, sodass klinikrelevante Themen bearbeitet werden.
Bitte akzeptieren Sie die Nutzung von Drittanbieter-Einbindungen mit einem Klick auf den folgenden Button, damit dieses Video abgespielt werden kann.
Wissenschaftler
Univ.-Prof. Dr. Björn Bachmann
Dr. rer. nat. Maria Notara
Dr. rer. nat.Thomas Clahsen
Dr. Deniz Hos
Dr. Franziska Bucher
Anna Lentzsch
Doktoranden
Yanghong Hou
Dr. Viet Nhat Hung Le
Alex Doulis
Ann-Charott Schneider
Anne Bukowiecki
MTA
Sara Behboudifard
Gabriele Braun
Marie-Luise Dreisow
Tim Gabriele
The role of lymphatic vessels in transplant rejection
Immune-mediated graft rejections remain the most common cause for graft failure after organ and tissue transplantation. There exists a great unmet medical need for pharmacologic strategies to promote graft survival without unduly compromising the health of the recipient.
The three structural components of the immune system allowing for immune responses against foreign tissue after transplantation are afferent lymphatic vessels (“afferent arm of the immune reflex arc”), regional lymph nodes (“central processing unit”) and efferent blood vessels (“efferent arm of the immune reflex arc”).
Lymphatic vessels allow the transport of antigen-presenting cells with foreign tissue antigens and soluble antigenic material to the regional lymph node and thereby constitute one of the earliest events in the immune-cascade leading to rejection. The precise relative importance of lymphatic vessels (“afferent arm”) versus blood vessels (“efferent arm”) for immune reactions after transplantation is yet unclear. But every solid organ or vascularized tissue transplantation is accompanied by angiogenesis and lymphangiogenesis across the wound edges. In fact, lymphatic vessels have been identified in allogeneic grafts after heart and kidney transplantation where their presence seems to be related to graft rejection.
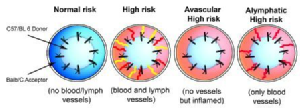
Corneal hem- and lymphangiogenesis occurring both prior to as well as after corneal transplantation significantly increase the risk for immune rejection: The rate of immune rejections in patient eyes with avascular graft beds is approximately 10%, whereas the rate of immune rejections increases in pre-vascularized, so called high-risk patient eyes to 50-100%. Lymphatic vessels and blood vessels override the so called immune privilege of the normally avascular cornea.
A combined modulation of hemangiogenesis and lymphangiogenesis by VEGF-TrapR1R2 after normal-risk corneal transplantation can improve graft survival in the murine model of corneal transplantation.
Blocking preferentially lymphangiogenesis over hemangiogenesis leads to inhibition of the induction of an immune response and at the same time blood vessels can still support the graft with nutrients and enable wound healing. The high-risk status of corneal allografts in vascularized host beds is defined by the lymphatic vessels and the preferential inhibition of lymphangiogenesis prior to transplantation is able to improve graft survival by interfering with sensitization and immune rejection.
Therefore it is important to identify ways to preferentially block lymphangiogenesis to promote graft survival.
Lymphatic vessels in dry eye diseases
Dry eye disease (DED) is the most common disease in ophthalmology. Up to 63% of the general public suffer from this malfunction of the precorneal tear film. A dysfunctional tear layer leads to ocular pain and serious vision impairment. DED can be induced by inflammatory diseases like autoimmune destruction of the lacrimal gland in Sjörgen´s Syndrom (5.1) as well as by desiccation, androgen deprivation or ageing. Independent of the underlying cause a secondary ocular surface inflammation occurs in most patients with DED. Recently we observed in a murine model of DED in Sjörgen´s syndrom (5.1) a spontenous and selective outgrowth of lymphatic vessels together with an increase of immune cells in the normally avascular cornea (5.3). This unexpected finding may suggest a novel pathomechanism in DED implicating lymphatic vessel-mediated sensitization.(5.2). The important role of lymphatic vessels in other immunological reactions in the cornea like corneal transplant rejection was recently approved by our group.
Genetic Heterogeneity of Lymphangiogenesis in Different Mouse Strains
Contrary to angiogenesis, where substantial progress in understanding the molecular mechanisms and regulation-pathways was gained in the last decades, lymphangiogenesis research was long hampered by the absence of specific molecular markers. This changed with the discovery of specific molecular markers, such as LYVE-1, Podoplanin, Prox1, and VEGFR-3 and various in vitro and in vivo models in the last few years.
However, available information concerning the field of lymphangiogenesis research still lags behind hemangiogenesis (e.g., in the field of genetic diversity). Genetic heterogeneity of angiogenesis in mice was first reported in 2000 by Rohan et al., who showed that—dependent on the genetic background—the response to growth factor–induced angiogenesis varies significantly between different inbred mouse strains. Strain-dependent differences were also published for the density and surface area of the resting limbal vessels after bFGF-induced neovascularization in the cornea. Genetic diversity influencing angiogenesis-regulating genes is implicated in altering the susceptibility to angiogenesis-dependent diseases like cancer, diabetic retinopathy, psoriasis, and others. In contrast to the evidence for genetic heterogeneity on angiogenesis, to date little is known in this context about lymphangiogenesis.
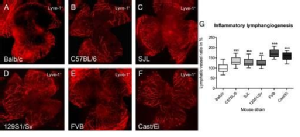
In different inbred and wild–derived mouse strains (Balb/cAnNCrl, C57BL/6NCrl, 129S1/SvImJ, SJL/JCrl, Cast/EiJ, FVB/NCrl), significant differences in the lymphangiogenic response were detected: the lymphvascularized area varied up to 2-fold between “low-responder” strains and the “high-responder” strains. Furthermore, the preexisting lymphatic vessels in the limbal region, which supports the avascular cornea with nutrients and is the origin of new blood and lymphatic vessels in the case of inflammation, are significantly different between low- and high-responders.
Anti-inflammatory and a specific antilymphangiogenic therapies induce different responsiveness to antilymphangiogenic treatments area in different mouse strains.
There are significant differences in the lymphangiogenic response of several mouse strains and underlying genetic factors influence the lymphangiogenic response. These considerations need to be taken into account when using different mouse strains to study lymphangiogenesis and can also explain different success of antilymphangiogenic treatments in tumor patients.
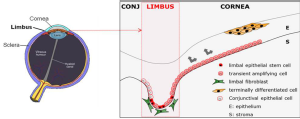
The role of limbal epithelial stem cells in maintaining corneal avascularity and in the pathogenesis of UV-induced pterygium
Corneal clarity is essential for vision. It’s avascularity is mediated by a dynamic balance of pro and anti-angiogenic signals. An intact corneal epithelium is necessary for transparency and refraction. This epithelial layer is constantly maintained by a limbal epithelial stem cell (LESC) population residing in the basal epithelial layer of the limbus. Dysfunction or depletion of limbal epithelial stem cells (limbal stem cell deficiency [LESCD]) results in persistent corneal inflammation and epithelial breakdowns, corneal surface conjunctivalisation and corneal neovascularisation. Conditions leading to LESCD and therefore neovascularization are chemical and thermal burns, inflammatory eye diseases, persistent hypoxia (contact lens wear) as well as genetic disorders such as aniridia (pax6 haplodeficiency). Patients with LESCD suffer from photophobia, reduced visual acuity and pain due to recurrent ocular surface defects. In severe cases LESCD can result to blindness.
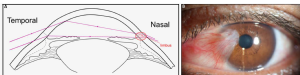
UV radiation (UVR) is damaging on various ocular structures leading to a reduction or even loss of visual function. The use of UV protecting eyewear such as sunglasses and more recently UV blocking clear lenses and contact lenses is recommended as a prophylactic measure against these harmful effects and there have been efforts to assess the effectiveness of UV-blocking eyewear more rigorously. The cornea is particularly susceptible to UV irradiation due to its natural transparency as well as to its shape which is contributing to a peripheral light focusing effect, affecting the nasal limbus where UV irradiation is 20-fold strongest (A). This is the typical site for the onset of pterygium, a non-cancerous growth of the cornea, usually bilateral, which is occupying the corneal equator (B). The pterygium breaks the limbal barrier which separates the cornea from the conjunctiva and centripetally invades the cornea surface. It is characterised by squamous hyperplasia and goblet cell hyperplasia. In advanced cases, the visual axis may be covered by vascularised opaque tissue thus leading to discomfort and decrease or loss of vision. Histological findings have led to the hypothesis that pterygium may be initiated by transformed basal limbal epithelial cells including LESCs which contribute to hyperplasia, tissue remodelling and vascularisation associated with the disease.
Our research focuses on the underlying molecular mechanisms by which LESCs contribute to corneal avascularity and how this is tampered following LESC niche exposure to UV. This research aims to understand the effect of UV to the cellular components of the limbal niche as well as to open up new treatment avenues against pterygium recurrence by developing preventative treatments against DNA damage and targeting immune cell recruitment-mediated prolymphangiogenesis.
- The effect of UVA and UVB on the limbal niche and pro-inflammatory and proangiogenic events
UV irradiation causes cumulative DNA damage and has an effect in the functionality of limbal epithelial cells and fibroblasts (viability, proliferation and migration). The expression of putative stem cell markers is also affected while proteomic assessment shows changes in pro-angiogenic, prolymph-angiogenic growth factors and cytokines as well as an upregulation of molecules which are known to contribute to immune cell recruitment (IFNγ, TNFα, MCP-1). - DNA damage in the limbal niche
LESCs are long cycling and therefore can accumulate DNA damage, direct or oxidative. For these studies, different types of DNA damage as well as strategies for its prevention and treatment are explored. - The protective effect of UV blocking contact lenses to the LESC niche
In collaboration with VISCTAKON® we investigate the potential benefits of a UV blocking contact lens against UV-induced changes in LESCs. This research has been supported by an independent research grant from J&J Vision Care. - The effect of different antiangiogenic treatments in LESCs
Pharmaceutical approaches of targeting pro-angiogenic and pro-inflammatory molecules are used clinically to treat and prevent corneal neovascularization. In these projects we investigate the effect of these treatments to the phenotype and functionality of LESCs.
Support
DFG (FOR 2240; Cu47/41-; 47/6-1); EU (Horizon 2020 Arrest Blindness; STRONG FP7; COST BM1302), Bayer Graduate Program Pharmacology
Bock F, Onderka J, Braun G, Schneider AC, Hos D, Bi Y, Bachmann BO, Cursiefen C. Identification of Novel Endogenous Anti(lymph)angiogenic Factors in the Aqueous Humor. Invest Ophthalmol Vis Sci. 2016 Dec 1;57(15):6554-6560. doi: 10.1167/iovs.15-18526. PMID: 27918829
Notara M, Refaian N, Braun G, Steven P, Bock F, Cursiefen C. Invest Ophthalmol Vis Sci. Short-Term Ultraviolet A Irradiation Leads to Dysfunction of the Limbal Niche Cells and an Antilymphangiogenic and Anti-inflammatory Micromilieu. 2016 Mar;57(3):928-39. doi: 10.1167/iovs.15-18343. PMID: 26943156
Notara M, Refaian N, Braun G, Steven P, Bock F, Cursiefen C.
Short-term uvb-irradiation leads to putative limbal stem cell damage and niche cell-mediated upregulation of macrophage recruiting cytokines. Stem Cell Res. 2015 Nov;15(3):643-54. doi: 10.1016/j.scr.2015.10.008. PMID: 26520427
Hos D, Cursiefen C. Lymphatic vessels in the development of tissue and organ rejection. Adv Anat Embryol Cell Biol. 2014;214:119-41.
Bucher F, Bi Y, Gehlsen U, Hos D, Cursiefen C, Bock F. Regression of mature lymphatic vessels in the cornea by photodynamic therapy. Br J Ophthalmol. 2014 Jan 10. doi: 10.1136/bjophthalmol-2013-303887
Hos D, Koch KR, Bucher F, Bock F, Cursiefen C, Heindl LM. Serum eye drops antagonize the anti(lymph)angiogenic effects of Bevacizumab in vitro and in vivo. Invest Ophthalmol Vis Sci. 2013;54:6133-42
Bock F, Rössner S, Onderka J, Lechmann M, Pallotta MT, Fallarino F, Boon L, Nicolette C, Debenedette MA, Tcherepanova IY, Grohmann U, Steinkasserer A, Cursiefen C, Zinser E. Topical Application of Soluble CD83 Induces IDO-Mediated Immune Modulation, Increases Foxp3+ T Cells, and Prolongs Allogeneic Corneal Graft Survival. J Immunol. 2013 Aug 15;191(4):1965-75
Cursiefen C, Regenfuss B, Hos D, Bucher F, Steven P, Heindl LM, Bock F. Anti(lymph)angiogenic preconditioning prior to keratoplasty. Klin Monbl Augenheilkd. 2013 May;230(5):500-4
Bock F, Maruyama K, Regenfuss B, Hos D, Steven P, Heindl LM, Cursiefen C. Novel anti(lymph)angiogenic treatment strategies for corneal and ocular surface diseases. Prog Retin Eye Res. 2013 May;34:89-124
Platonova N, Miquel G, Regenfuss B, Taouji S, Cursiefen C, Chevet E, Bikfalvi A Evidence for the interaction of fibroblast growth factor-2 with the lymphatic endothelial cell marker LYVE-1. Blood. 2013 Feb 14;121(7):1229-37
Maruyama K, Nakazawa T, Cursiefen C, Maruyama Y, Van Rooijen N, D'Amore PA, Kinoshita S. The maintenance of lymphatic vessels in the cornea is dependent on the presence of macrophages. Invest Ophthalmol Vis Sci. 2012;53:3145-53
Cloutier F, Lawrence M, Goody R, Lamoureux S, Al-Mahmood S, Colin S, Ferry A, Conduzorgues JP, Hadri A, Cursiefen C, Udaondo P, Viaud E, Thorin E, Chemtob S. Anti-angiogenic activity of Aganirsen in non-human primate and rodent models of retinal neovascular disease following topical administration. Invest Ophthalmol Vis Sci. 2012 Mar 9;53(3):1195-203
Koenig, Y., et al., Angioregressive Pretreatment of Mature Corneal Blood Vessels Before Keratoplasty: Fine-Needle Vessel Coagulation Combined With Anti-VEGFs. Cornea, 2012.
Steven, P., et al., Intravital two-photon microscopy of immune cell dynamics in corneal lymphatic vessels. PLoS One, 2011. 6(10): p. e26253.
Hos, D., et al., Blockade of insulin receptor substrate-1 inhibits corneal lymphangiogenesis. Invest Ophthalmol Vis Sci, 2011. 52(8): p. 5778-85.
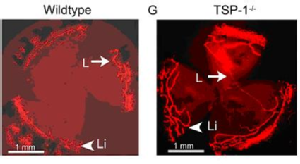
Cursiefen, C., et al., Thrombospondin 1 inhibits inflammatory lymphangiogenesis by CD36 ligation on monocytes. J Exp Med, 2011. 208(5): p. 1083-92.
Hos, D., et al., Suppression of inflammatory corneal lymphangiogenesis by application of topical corticosteroids. Arch Ophthalmol, 2011. 129(4): p. 445-52.
Bock, F., B. Regenfuss, and C. Cursiefen, [Antiangiogenic therapy at the ocular surface: when, what and why?]. Ophthalmologe, 2011. 108(3): p. 230-6.
Regenfuss, B., et al., Genetic heterogeneity of lymphangiogenesis in different mouse strains. Am J Pathol, 2010. 177(1): p. 501-10.
Dietrich, T., et al., Cutting edge: lymphatic vessels, not blood vessels, primarily mediate immune rejections after transplantation. J Immunol, 2010. 184(2): p. 535-9.
Cursiefen, C., et al., GS-101 antisense oligonucleotide eye drops inhibit corneal neovascularization: interim results of a randomized phase II trial. Ophthalmology, 2009. 116(9): p. 1630-7.
Zimmermann, P., et al., Tumour-associated lymphangiogenesis in conjunctival malignant melanoma. Br J Ophthalmol, 2009. 93(11): p. 1529-34.
Koenig, Y., et al., Short- and long-term safety profile and efficacy of topical bevacizumab (Avastin) eye drops against corneal neovascularization. Graefes Arch Clin Exp Ophthalmol, 2009. 247(10): p. 1375-82.
Regenfuss, B., et al., [Topical inhibition of angiogenesis at the cornea. Safety and efficacy]. Ophthalmologe, 2009. 106(5): p. 399-406.
Bachmann, B.O., et al., Transient postoperative vascular endothelial growth factor (VEGF)-neutralisation improves graft survival in corneas with partly regressed inflammatory neovascularisation. Br J Ophthalmol, 2009. 93(8): p. 1075-80.
Bock, F., et al., Safety profile of topical VEGF neutralization at the cornea. Invest Ophthalmol Vis Sci, 2009. 50(5): p. 2095-102.
Regenfuss, B., et al., Corneal (lymph)angiogenesis--from bedside to bench and back: a tribute to Judah Folkman. Lymphat Res Biol, 2008. 6(3-4): p. 191-201.
Bock, F., et al., Improved semiautomatic method for morphometry of angiogenesis and lymphangiogenesis in corneal flatmounts. Exp Eye Res, 2008. 87(5): p. 462-70.
Hos, D., et al., Age-related changes in murine limbal lymphatic vessels and corneal lymphangiogenesis. Exp Eye Res, 2008. 87(5): p. 427-32.
Hos, D., et al., Inflammatory corneal (lymph)angiogenesis is blocked by VEGFR-tyrosine kinase inhibitor ZK 261991, resulting in improved graft survival after corneal transplantation. Invest Ophthalmol Vis Sci, 2008. 49(5): p. 1836-42.
Bachmann, B.O., et al., Promotion of graft survival by vascular endothelial growth factor a neutralization after high-risk corneal transplantation. Arch Ophthalmol, 2008. 126(1): p. 71-7.
Bock, F., et al., Bevacizumab (Avastin) eye drops inhibit corneal neovascularization. Graefes Arch Clin Exp Ophthalmol, 2008. 246(2): p. 281-4.
Bock, F., et al., Blockade of VEGFR3-signalling specifically inhibits lymphangiogenesis in inflammatory corneal neovascularisation. Graefes Arch Clin Exp Ophthalmol, 2008. 246(1): p. 115-9.
Dietrich, T., et al., Inhibition of inflammatory lymphangiogenesis by integrin alpha5 blockade. Am J Pathol, 2007. 171(1): p. 361-72.
Bock, F., et al., Bevacizumab as a potent inhibitor of inflammatory corneal angiogenesis and lymphangiogenesis. Invest Ophthalmol Vis Sci, 2007. 48(6): p. 2545-52.
Bock, F., et al., [Inhibition of angiogenesis in the anterior chamber of the eye]. Ophthalmologe, 2007. 104(4): p. 336-44.