Copyright: Wolters Kluwer Health, Inc.
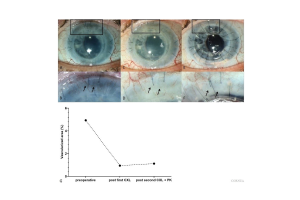
Copyright: Wolters Kluwer Health, Inc.
Kontakt
Die Uniklinik Köln steht rund um die Uhr für die Versorgung von Notfallpatienten zur Verfügung.
In lebensbedrohlichen Situationen / Notruf
Ärztlicher Notdienst
Notfallambulanz für Augenheilkunde
Anfahrtsadresse für Navigationsgeräte
Kerpener Straße
62
50937
Köln
Augenklinik Gebäude 34
Zufahrt über Gleueler Straße
Corneal transplantation is the most common form of tissue transplantation and the prime cure for corneal blindness. The Department of Ophthalmology in Cologne performs about 10% of all corneal transplantations in Germany. Corneal transplantation normally is very successful. This refers to so-called „low-risk“, i.e. avascular and non-inflamed corneas. In contrast, pathological ingrowths of blood and even more so lymphatic vessels significantly increases the risk for immune rejection after subsequent, so called „high-risk“ transplantation. This affects about 10% of all transplantations in Germany and more than 75% of transplantations worldwide.
The research group of Prof. Dr. Claus Cursiefen and Prof. Dr. Felix Bock deals with the mechanism of graft rejection after corneal transplantation. The focus is mainly on the role of blood and lymphatic vessels in mediating these rejections and on novel ways to promote graft survival by targeting pathologic corneal blood and lymphatic vessels. Our research group uses various primarily murine models of high-risk settings and murine corneal transplantation as well as other techniques to investigate both the formation and interaction of lymphatic vessels with other cells of the immune system and ways to influence or prevent the growth and interactions. Applied methods are various ophthalmologic mouse models from surgical to genetic models. Furthermore, we work with immunohistochemical as well as molecular biological methods like qPCR and Westen blot, FACS analysis, cell culture and intravital multiphoton microscopy in collaboration with the ocular surface group.
The group is in close contact with the clinic and is therefore strongly involved in the process "From Bench to Bedside and back", so that clinically relevant topics are worked on. As an example, we identified clinically invisible corneal lymphatic vessels as prime inducers of graft rejection after transplantation and based thereon developed the novel concept of a „temporary pretransplant lymphangioregression of the recipient corneal bed to promote subsequent high-risk corneal transplantation“. This was established first preclinically in our lab and now is in pilot clinical testing in a phase I clinical trial funded by the BMBF (German Ministry of Reserach; see below).
Research is conducted in close collaboration with local partners in Cologne such as in the CMMC (Center for Molecular Medicine Cologne) and CECAD (Cluster of Excellence), but also with European and international partners (www.restorevision-project.eu, www.aniridia-net.eu).
Bitte akzeptieren Sie die Nutzung von Drittanbieter-Einbindungen mit einem Klick auf den folgenden Button, damit dieses Video abgespielt werden kann.
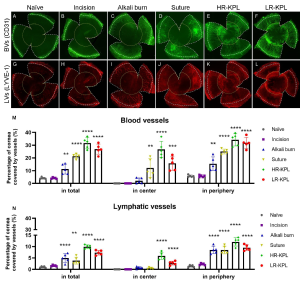
Personalized Lymphvascular- and Immunomodulation in High Risk Corneal Transplantation (Joined Project AG Cursiefen/AG Bock within DFG RU 2240; 2015-2023)
The healthy cornea is the “windscreen” of the eye. Pathologic insults like chemical or thermal burns, hypoxia, transplant rejection or trauma lead to blindness and to a so called “high risk situation” with increased rejection rates after subsequent corneal transplantation.
All these insults cause different immunological tissue responses. We could e.g. show that a suture induced inflammation causes the ingrowth of both blood and lymphatic vessels whereas corneal trauma only causes an isolated lymphangiogenic response. Trauma and burns induce primarily an innate immune response whereas corneal grafts induce adaptive, allospecific immunity.
Nonetheless, irrespective of the underlying cause, so far corticosteroids have remained the mainstay of immunomodulatory therapy after i.e. high risk penetrating corneal transplantation, but only 50% of the rejection episodes respond to that treatment.
Personalized medicine, already approaching clinical routine in oncology, has not conquered ophthalmology yet.
Recently, we could show that the different types of corneal injury caused significantly different hem- and lymphangiogenic responses. Furthermore, the infiltration of corneal macrophages, dendritic cells, neutrophils, and MHC II+ cells varied significantly in different high-risk settings. In conclusion, the murine high-risk settings caused by different underlying pathologies varied significantly in their (lymph)angiogenic and inflammatory cell patterns. (Zhang W, Schönberg A, Bassett F, Hadrian K, Hos D, Becker M, Bock F*, Cursiefen C.*Different Murine High-Risk Corneal Transplant Settings Vary Significantly in Their (Lymph)angiogenic and Inflammatory Cell Signatures. Invest Ophthalmol Vis Sci. 2022).
Our data demonstrate that different “immune- and lymphvascular signatures” indeed exist and that they depend on the underlying injury. These signatures may need specific and disease-oriented novel therapeutic approaches to promote graft survival, i.e. customized immune modulatory and lymphangioregressive approaches.
EU Horizon Project RESTORE VISION: Novel anti-inflammatory and lymphangiomodulatory therapies for rare ocular surface diseases (such as CNV, Aniridia, Ocular Pemphigoid etc.; 2023-2027)
Complementary, we are also part of the EU Horizon action RESTORE VISION, in which we are mainly involved in the design and operation of clinical studies of known therapies for rare eye diseases (REDs). These REDs are a major cause of visual impairment and blindness in Europe, affecting patients of all ages. The RESTORE VISION Consortium identified a group of REDs all affecting the cornea and ocular surface that cause severe vision impairment and blindness and have inadequate treatment options today.
Current management is often prohibitively expensive, has low efficacy and leads to debilitating side effects, pointing to a critical medical problem and area of unmet medical need. New therapeutics with game-changing potential will be evaluated for the first time. The pioneering ‘streams’ approach of the consortium is based on the repurposing of 6 existing drugs and the development of 3 new compounds, all with solid preliminary data showing remarkable effects in restoring the cell physiology, immune, avascular, neural and signalling environment in the cornea. This innovative approach shortcircuits lengthy and complex regulatory and drug development processes, ensuring rapid translation into the clinic.
Focus is amongst others on preclinical work and pilot clinical trials on:
A. Antisense Oligonucleotide eye drops against IRS-1 and UV-based crosslinking against inflammatory neovascularisation in a murine model of Aniridia and in patients affected by Aniridia
B. Targeting IRS-1 signalling as adjuvant therapy in regressing pathologic corneal blood and lymphatic vessels in murine models and patients
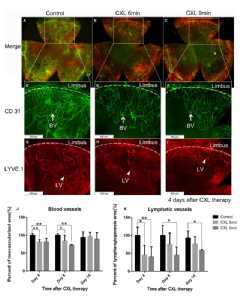
Corneal Crosslinking and Fine-needle diathermy as (Lymph)Angioregressive treatments to improve graft survival (DFG Cu 47/12-1; 15-1; 2023-2025)
Besides pharmacological strategies, we and others have identified lymphangioregressive (and immunodampening) strategies to prepare the high-risk recipient bed for future transplantations (Clahsen et al., PRER, Prog Retin Eye Res. 2023 Feb 7:101157. doi: 10.1016/j.preteyeres.2022.101157). Besides FND (fine needle diathermy) and PDT (photodynamic therapy; [25], UVA-light based Corneal Crosslinking (CXL) was shown to not only regress pathologic corneal blood and lymphatic vessels [26], but to also induce reduction of corneal antigen presenting cells (APCs) and most importantly to promote subsequent graft survival after high-risk transplantation [26]. In fact, all three approaches led to temporary regression of pathologic corneal blood and lymphatic vessels as well as alteration/depletion of corneal resident APCs before transplantation. Subsequently, they promoted graft survival in the murine high-risk model. Preliminary clinical data from patients undergoing high-risk transplantation suggest that UVA-based corneal crosslinking also regresses pathologic corneal neovascularization in patients [19]. Even more so, a retrospective mono-centric pilot study on pre-transplantation FND in high-risk patients demonstrated good tolerability and relatively good subsequent corneal graft survival [22]. Whether the beneficial effect on graft survival is due to (lymph)angioregression, depletion and alteration of APCs or both remains unclear.
The phenomenon of UV-light mediated alteration of APCs is well known e.g. from UVB irradiation of the skin [27] and previous studies of UVB effects in experimental cornea studies. It is still unknown what the optimal strategy is to down regulate the immune response and regress lymphangiogenesis in high-risk recipient beds and whether that varies between different underlying diseases.


BMBF-funded pilot clinical trial - UV light-mediated corneal crosslinking as a (lymph)angioregressive pretreatment to improve graft survival after subsequent high-risk corneal transplantation (2023-2027)
Based on the above mentioned data, the BMBF-funded prospective randomized multi-center trial CrossCornealVision testing pre-transplantation UVA-based CXL as a new method to promote high-risk
Recently, UV-based corneal crosslinking (CXL) using Riboflavin as photosensitizer, a technique used in the clinic to stabilize corneas, was shown to also destroy corneal vessels both experimentally and in pilot clinical data. In the CrossCornealVision Study we test the novel concept of “(lymph)angioregressive preconditioning” of high-risk eyes using CXL to promote graft survival in a prospective, randomised multicenter exploratory clinical trial. Prior to high-risk transplantation, patients are randomised into CXL or no pretreatment (control). Then, incidence of graft rejection episodes and safety are evaluated. This will be the first improvement to promote graft survival in high-risk transplants for decades, and will significantly improve vision and quality of life in patients suffering from corneal blindness and help designing a later confirmatory trial.
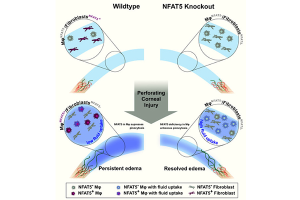
Targeting the osmosensitive transcription factor NFAT5 in acute and chronic corneal edema: ZMMK Projekt (2023-2025): Dr. Karina Hadrian, PD Dr. Deniz Hos, Prof. Cursiefen, Claus
The cornea is the transparent avascular outer barrier and major refractive element of the eye. Loss of corneal transparency, e.g. due to dysfunction of corneal endothelial cells resulting in corneal swelling (edema), leads to corneal blindness and is the second most common cause for blindness worldwide. The only possible treatment so far is corneal transplantation, resulting in more than a million people suffering from corneal blindness due to shortage of donor corneas worldwide. Due to shortage of tissue donors, only 1 in 70 patients can be cured. Thus, non-surgical approaches to reduce corneal edema would be of great therapeutic value to treat corneal blindness. In this context, we recently discovered a novel suppressive role for the osmosensitive transcription factor nuclear factor of activated T cells 5 (NFAT5; or tonicity-responsive enhancer binding protein; TonEBP) in corneal edema resorption, thereby identifying a novel potential therapeutic target to non-surgically combat edema-induced corneal blindness. Aim of this work is to translate these findings into a clinically applicable direction by therapeutically modulating NFAT5 in the cornea in mouse models of acute and chronic corneal edema. Furthermore, this study will investigate the impact of modulating NFAT5 on graft survival after corneal transplantation.
DFG Research Unit 2240 (www.for2240.de): P1 (CC; FB); DFG Cu 47/12-1, Cu 47/15-1; BO4489/1-2, HO 6270/1-1, BO 4489/3-1; Köln Fortune (FB) EU Restore Vision (www.restorevision-project.eu; CC, FB); BMBF CrossCearClear (CC, MM, JW); Center for Molecular Medicine Cologne (www.cmmc-unikoeln.de; CC, KH, DH); RETINOVIT Foundation Cologne (CC).